If I review the term Haber Process and accompany it with descriptive words such as industry, ammonia, nitrogen, fertilizer and energy consumption, it is almost inevitable that most will come to mind the image of factories working by the piece, releasing columns of smoke into the air through its long chimneys and, in short, polluting the environment. And some of that -or a lot- there is, of course. But, paradoxically, it also has positive effects, fundamental in fact, and I'm not just referring to the economy or the jobs it generates.
To be exact, this process is called Haber-Bosch because its creators were Fritz Haber and Carl Bosch, two German chemists who collaborated hand in hand in 1910 to develop a system for producing ammonia through a reaction of atmospheric dinitrogen and dihydrogen , induced by a metal catalyst at high temperature and pressure.
The invention was of great importance due to the difficulty that existed until then in producing ammonia on an industrial scale, since the systems that had existed until then, such as the Birkeland-Eyde or the Frank-Caro, were not efficient.

And why was so much ammonia necessary? Currently we would say that because it is the basic component of fertilizers. However, in those first decades of the 20th century the objective was the production of explosives and ammunition. In this sense, Germany had a special interest due to the arms race in which it was immersed in the face of the increasingly inevitable war that was approaching and that, when it finally broke out, meant a limitation of raw materials for the German arms industry, since the Allies blocked their imports of saltpeter from Chile (the companies were British).
In fact, the growing demand for ammonia and nitrates was not only Teutonic but worldwide since the previous century. As we said before, there is an important source of nitrogen, our own air, in which this element constitutes 87%, but it is so stable that it is difficult to make it react with other chemical products; that is why achieving his conversion was a challenge for science. Fritz Haber manufactured a high pressure instrument with which he managed to produce ammonia from air drop by drop and in 1909 he officially presented it.

The Teutonic company BASF acquired the invention to apply it on an industrial scale, being Carl Bosch the one in charge of making this adaptation the following year and beginning the manufacture of ammonia in 1913. The success was evident when twenty tons per day began to be obtained, which allowed produce ammunition in the desired quantity. Thus, Germany was able to face the First World War without depending on anyone while sales of Chilean saltpeter, by the way, fell by two thirds and its price plummeted.
Once the war was over, possible prejudices were set aside and academic justice was imposed:the works of Haber and Bosch were part of the merits, later expanded with research on other topics, which led to them being awarded the Nobel Prize in Chemistry in 1918 and 1931, respectively.
It must be said that Haber, a proud patriot, had participated in the development of the dichlor gas used in the Second Battle of Ypres and the Eastern Front; instead, he refused to work for the Nazis because, although a Christian, he was of Jewish descent (ironically, they used his gases for the Final Solution). Bosch didn't want to deal with them either.

Times have changed and today the main use of the Haber Process is the production of ammonia for use as fertilizers. To do this, nitrogen and hydrogen (this is obtained from methane in natural gas in a nickel catalyst that separates carbon and hydrogen atoms) are combined at a temperature of about 400º or 500º and between 150 and 300 atmospheres of pressure. The original general catalyst, osmium, was later replaced by uranium, which is more abundant, but now a cost-reducing magnetite (oxidised iron powder)-based catalyst is used.
Until Haber devised his system, industrial fertilizers were made by the Cyanamide Process, invented in 1898 by Adolph Frank and Nikodem Caro (hence also called the Frank-Caro Process). It consisted of an exothermic reaction (that is, energy-producing) of calcium carbide with nitrogen that was carried out in large steel cylinders heated to a thousand degrees of temperature, which caused two things:on the one hand, carbon; on the other, nitrolim (a solid mixture of calcium cyanamide, white and odorless crystals that were the base of fertilizers).
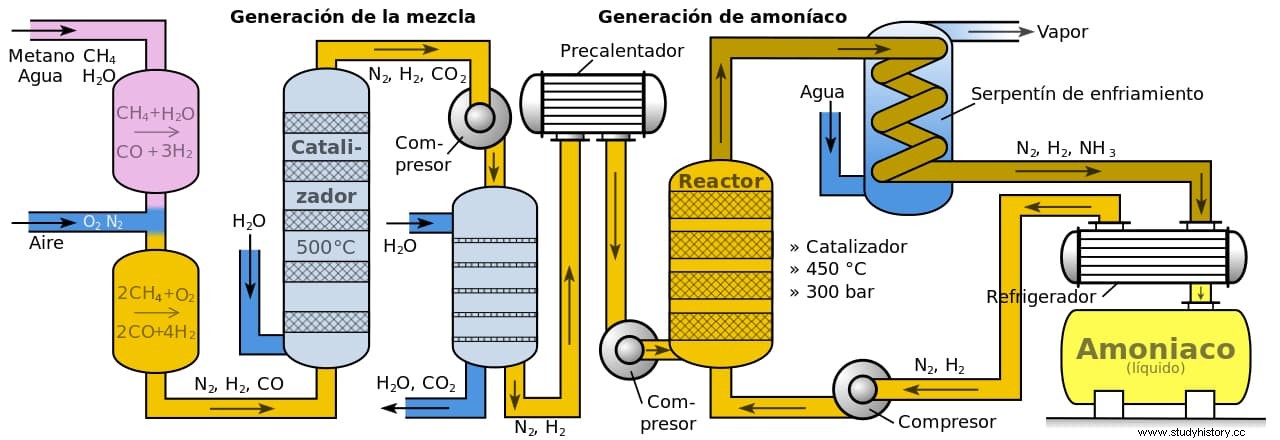
The problem was that this method required the consumption of huge amounts of electricity and a huge workforce, so the diffusion of the Haber Process relegated it to the background. It is still used but it is the other one that really takes the lion's share in the industrial sector, with a production of about 450 million tons per year. This requires, on the contrary, the use of between 3% and 5% of the world's natural gas, which approximately represents 1% to 2% of the planet's energy supply.
The data could seem totally negative, especially if other derivatives are added, such as the fact that only 17% of the ammonia manufactured is consumed, leaving the rest in the earth, the air or the water; something that, according to some studies, has altered the natural nitrogen cycle. Thus, more than half of the ammonia and nitrates end up spreading through the soil and, through runoff, pass into rivers and seas, affecting their biological habitats by providing them with an excess of nutrients. That makes algae and bacteria proliferate as they are so well fed, consuming the oxygen that other species need. This is known as eutrophication.
In fact, something similar happens in the atmosphere, altering its balance, since excess nitrogen enriches the ozone in the troposphere and reduces that in the stratosphere. Seeing the glass half full, the higher percentage of nitrogen in the air favors the capture of CO2 in large forest masses. Moreover, to all these drawbacks we can add a much more positive second reading, related to the growth and food of the world population:the use of these fertilizers has quadrupled the productivity of farmland in the last century, allowing they occupy less than 15% of the total surface of the Earth.
This has had repercussions, it is also true, in a demographic explosion that has increased the number of inhabitants from 1,600 million in 1900 to the impressive 7,000 million of today. But thanks to fertilizers, agriculture and livestock have reached such important levels that they support a third of the human beings on the planet, otherwise the land would not be productive enough.
