History of studying radioactive and nuclear energy
The first significant studies of nuclear radioactivity emerged during what Lawrence Badash refers to in Rutherford and Boltwood:Letters on Radioactivity (1969) as the scientific revolution of the 1980s that "changed the face of science in a little more than a generation, in stark contrast to the one hundred and fifty years that included the contributions of Copernicus, Kepler, Galileo, Descartes and Newton" (1). In particular, the efforts of people like Ernest Rutherford (1871-1937), Henri Becquerel (1852-1908) and Marie Sklodowska Curie (1867-1934) marked decades of exponential scientific success rates.

The spread of scientific innovation in the years before World War II (1939-45) metastasized into the discoveries of nuclear physics, making the creation of the world's first nuclear weapon a tangible attempt. By and large, the findings in nuclear physics through the 1940s compliment the insights of the researchers who worked through the 1890s, while outlining the ways in which the development of new weapon technologies caused a violation of the political climate in the 1940s to the broader scientific field. . By discussing the work and actions of Klaus Fuchs (1911-88) -German-born socialist became communist, physicist on the Manhattan project and infamous Soviet spies-we can argue that the specific innovation of nuclear fission with uranium isotopes (U 238 and you 235 ) during World War II, the subsequent Tube Alloy project in Britain, and the French heavy water production that soon followed, was provoked by an Allied effort to claim a note on international nuclear power to emit the threat from the Axis powers.


Rutherford's first reassessment of the atomic structure marked the early incarnation of nuclear physics. Perhaps his most influential experiment was gold foil and alpha particles. According to JJ Thomson's (1856-1940) 'plum pudding' model, the atom was a positively charged particle with smaller electron particles built into it. Based on this model, Rutherford postulated that if he shot alpha particles from a lead box, against a thin sheet of gold foil, they would necessarily pass through the foil linearly, while a small number would experience a small departure by traveling along some of the electron particles in the gold foil atoms ( Au). Initially, his hypothesis was confirmed, but he sought to inquire further whether the particles would really move through the gold foil - with only small deflections - if he placed a detector screen around the foil, thus leaving a small opening for the entrance to the alpha particles coming out of the lead box. . Consequently, Rutherford observed that about one in every 20,000 alpha particles did not pass through the foil, but rather were deflected back onto the screen. He concluded that the particles must have been deflected by a very small but heavy mass inside the atom. This mass must have contained protons that derived the positively charged alpha particles.
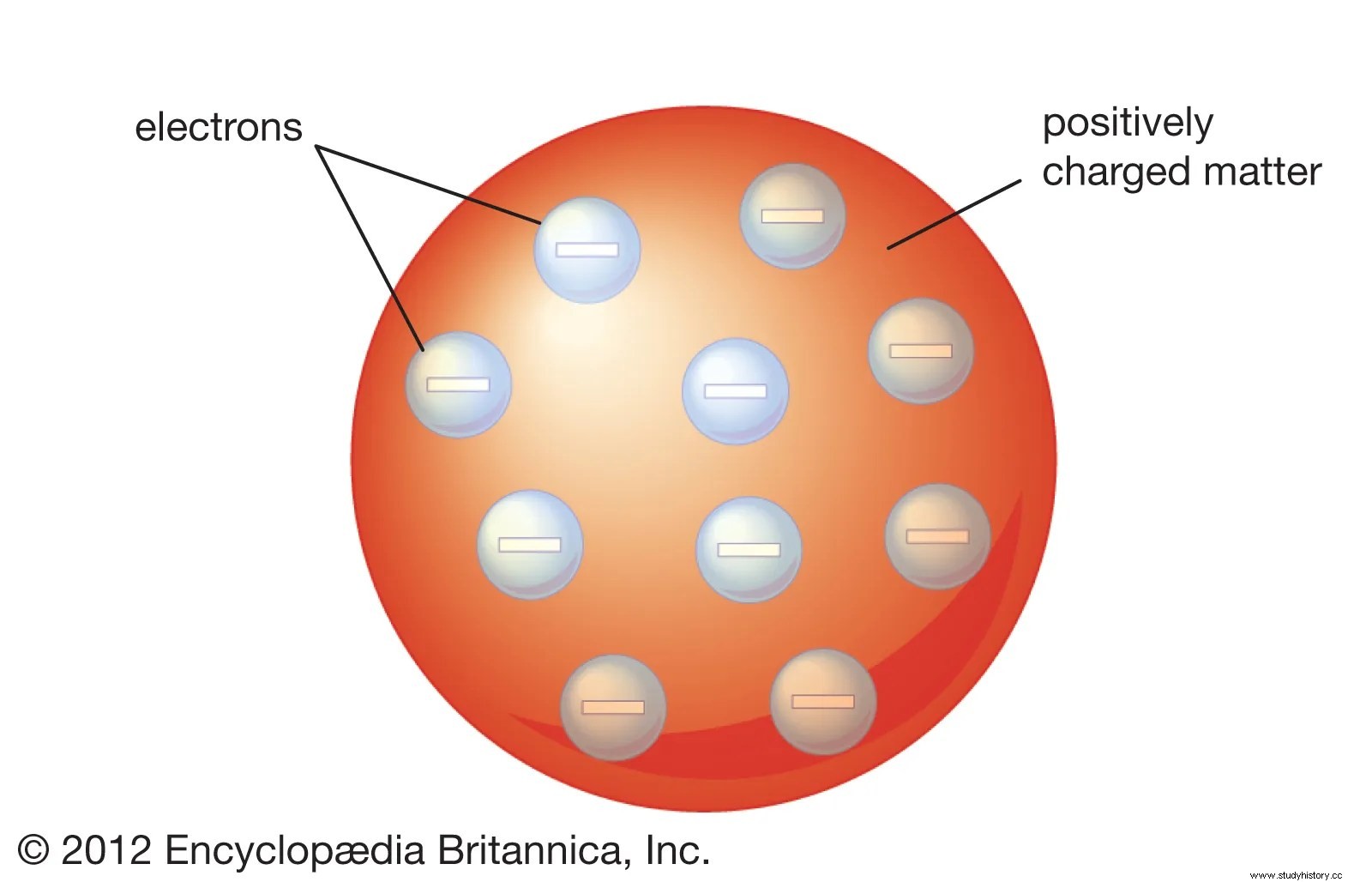
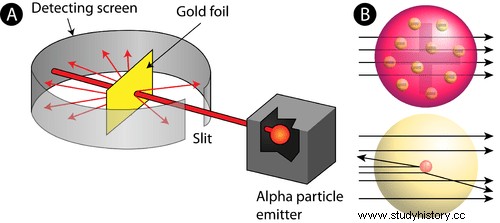
Rutherford then constructed the first modern model of the atom in which his theory of the atomic nucleus described the small, positive mass at the center of each atom. He further concluded that atoms actually contained electrons, but those that were largely spaces (Khan Academy). Consequently, his model revolutionized the notion that the atom consists largely of empty space and marked the early stages of studies in nuclear physics and radioactivity.
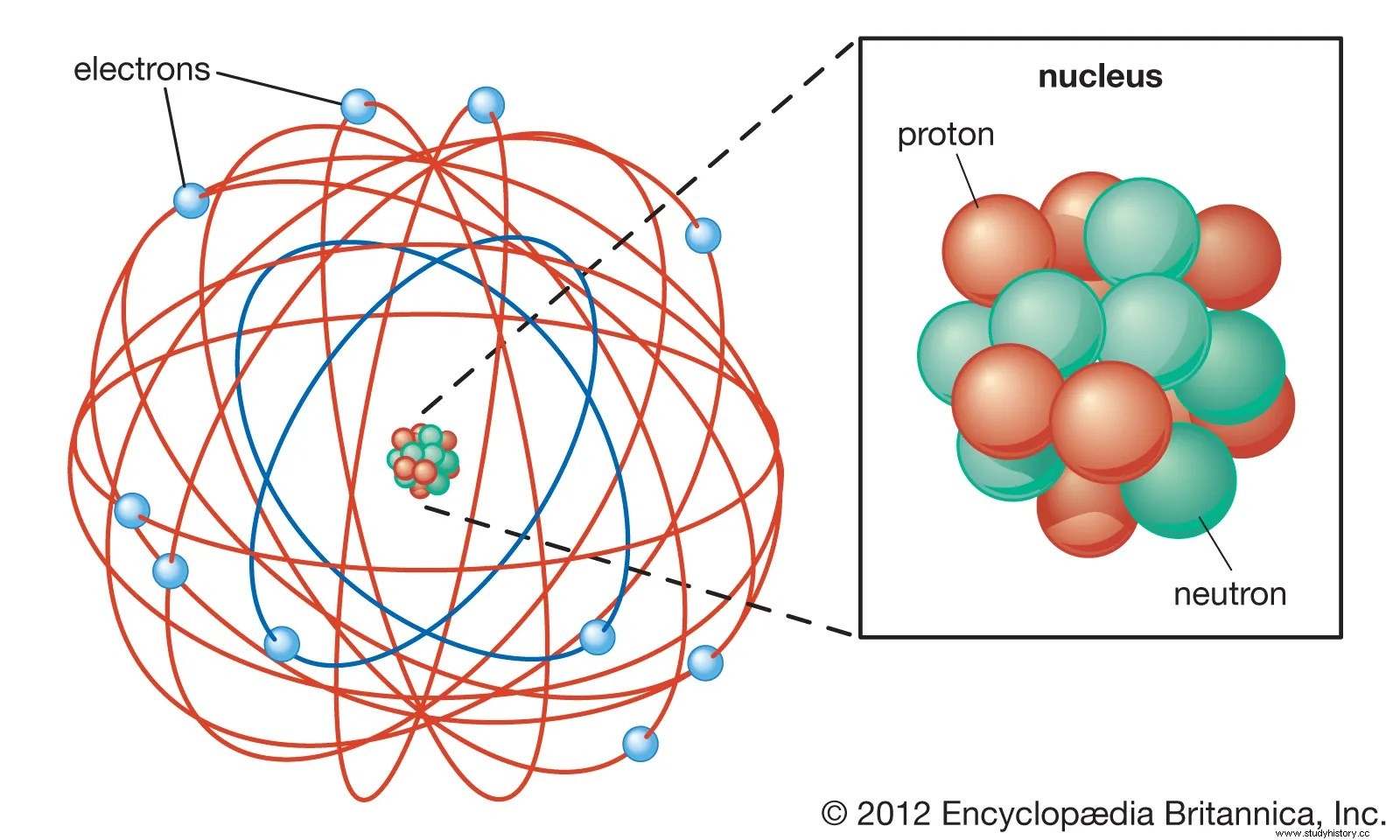
Based on Rutherford's findings, a series of experiments unfolded and then influenced the discovery of nuclear fission in 1938 (Clarke 12). Two decades after Rutherford discovered the nucleus, James Chadwick (1891-1974) discovered the neutron particle (Map 15). This new discovery contributed to Frederick Soddy's discovery of isotopes, Danish Niels Bohr (1885-1962) then added the neutron to his model of a "cloud of electrons surrounding a nucleus of protons", American Harold Urey (1893-1981) who discovered deuterium- essential for the French production of heavy water and Italian Enrico Fermis (1901-1954) initial experiment with the use of uranium particle separation in 1934 (Clark 37). As a consequence of this series of physical innovations, Otto Hahn (1878-1968) and Fritz Strassman (1902-80) gathered the diversity of close research that had been devoted to their field since Rutherford's first discovery of the nucleus and carried out the first uranium fission in the autumn. 1938 (Map 15).
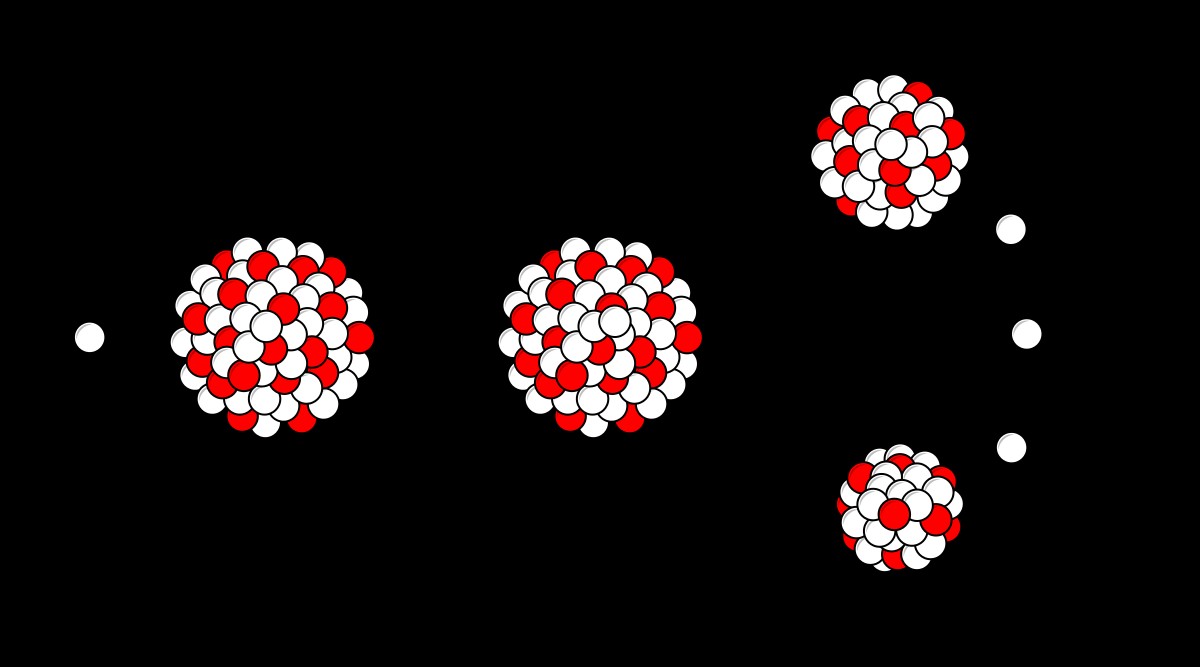
Hahn and Stressman "bombarded" the slow-moving neutrons of uranium (U) and found that it could split to produce anatomically lighter elements such as "barium, actinium and lanthanum" (Clark 36). In addition, the fission observed the release of enormous amounts of energy, suggesting the first possibility of nuclear power. In January 1939, Otto Frisch (1904-79) and his aunt, Lise Meitner (1878-1968), concluded that Hahn and Strassman had discovered a tangible and revolutionary new source of power and created the concept. nuclear fission due to the similarity of the process with the separation of bacteria in chemistry (Clarke 13).
Needless to say, the evolution of nuclear physics and uranium fission did not follow a linear path, nor did they permeate the scientific domain with the discovery of a unitary theory, discovered by a single individual. On the contrary, they were characterized by international interest from a number of researchers to participate in the development of the physical sciences. It is crucial to note that in the initial stages of development, the motive for research in the special field of nuclear physics was ignited by curiosity (Kelly 283). In fact, it was only the terror of World War II that the scientific inquisition for nuclear power research became the calculated, political production of what would become the world's deadliest weapon.

Extensive studies of nuclear uranium through the late 1930s concluded that only specific types of isotopes produced the radioactive energy that would be desirable for the completion of a nuclear weapon. In 1938, Niels Bohr had confirmed that U of the two uranium isotropic elements 238 was produced in large quantities during fission, but was usually absorbed by the neutrons after decomposition, thus not giving significant releases of energy. The far less abundant U 235 however, isotope produced the energy that would be needed on an "industrial scale", but the problem was that this type of specific isotropic separation had never been performed on such a large scale before (Clark 39). This conflict would be the main focus of the first studies of nuclear technology at both the Tube Alloy project in the United Kingdom, as well as the nuclear research studies at the Collège de France in the 1940s.
Although a post-apocalyptic film about a post-atom world, this film provides a good overview of the Allied efforts of World War II.

The first curiosity to observe the fission of U 235 eventually became more about the broader state of political anxiety provoked by power in Nazi Germany. By 1940, the threat from the Axis powers had shaken large parts of Europe, with a growing international interest in nuclear power being concentrated quite prominently in Britain. Since it was clear that Hitler's forces were planning a complete takeover of Europe, Britain and its allies developed a political plan designed to liberate the continent from its Nazi stronghold by means of an indestructible weapon.

The first evidence of the need for government intervention in relation to scientific findings related to nuclear power can be observed at the establishment of the Tube Alloy project in the United Kingdom (1941). The project was a government-led initiative based on the Maud Committee's report, which describes "the feasibility of producing an atomic bomb" (Kelly 50). The final report, prepared by Chadwick, predicted that an atomic bomb could be made in time to defeat the Axis powers, but that this would require specific resources and procedures. The main conclusions of the report were:
- The scheme for a uranium bomb is practically possible and is likely to lead to decisive results in the war.
- Work must continue on the highest property and on the increasing scale necessary to obtain the weapon in the shortest possible time.
- European powers' cooperation with America should be continued and expanded in the field of experimental work (Kelly 55).
The conclusions of the Maud report represent the first violation of the political world into the scientific field, with the British government recognizing the need to promote a weapon that can ensure the destruction of Axis forces. Another key aspect of the report is that it bypassed nationalist borders, and which clearly describes the need for cooperation with the United States. The report eventually gave rise to the establishment of the Tube Alloy project:an official research and development project dedicated to creating a working atomic bomb. The project served primarily as a precursor to the larger initiative of the Manhattan Project, which produced Little Boy and Fat Man (August 6, 9, 1945). The main goal of the Tube Alloy project was therefore to "produce raw material for the bomb" and "start with a development plan that would be the prototype" (Clark 157). It differed from the initial efforts of the Maud Committee, which had been primarily research-based, and marked an established Anglo-American effort for "scientific cooperation" (Clark 160).

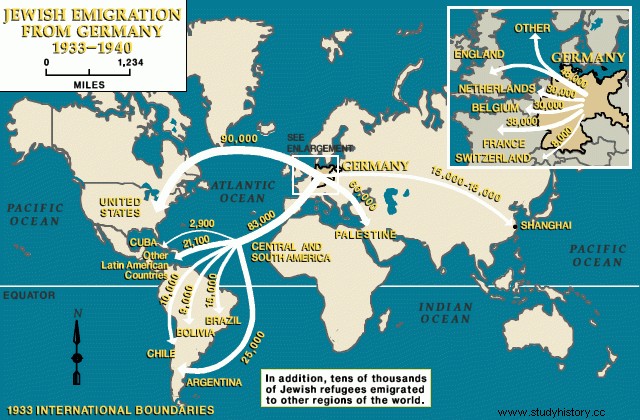
Naturally, the researchers involved in this work were pleased with the opportunity to disseminate their ideas on a more international scale. After all, the project required the efforts of a number of researchers - based on the strength of their skills, rather than their nationalities - as a notorious example is Klaus Fuchs. A German-born immigrant to England whose family had fled Nazi intervention because of their socialist leanings, Fuchs was considered a security risk by the get-go (Williams 39-40). However, Fuchs, "like other German havens and communists [...] was soon to become a valuable British resource in the war against Hitler". In fact, a large number of German refugees seeking asylum in Britain remained loyal to their socialist and communist sympathies so that the British government was "aware of this fact, but long-standing traditions of political asylum, together with anti-fascist sentiment from today, led them to ignore or tolerate the radicals in their midst ”(Williams 20). Even more than that, the government knew that if they wanted to create a weapon that would defeat the Axis powers, they would need to secure as many resources as possible, including scientists from different backgrounds.

Fuchs' father, Emil (1874-1971), was a devout socialist supporter and Protestant pastor whose political ideologies absolutely influenced all of his children. Unlike many of the elite, the literary class of Germany, in the years that followed the fall of the Weimar Republic (1919-33), "communism seemed to be the only force powerful enough to stop Hitler's rise" (Williams 13, Crew 19). ). After studying at universities in Germany and England, Klaus remained loyal to the German Communist Party (KDP) and was "convinced that the Communist Party line was right" (Williams 14-16). Despite fears of radical left-wing policies in the West, the Allies could not deny their broader conviction to put an end to the war. As such, in 1941 Fuchs became an interesting figure for the Tube Alloy project and became part of "the stream of German refugee scientists who took with them the physics needed to build an atomic bomb" (Williams 35).

One reason the Allies were so determined to quickly produce a nuclear weapon was because they were almost certainly convinced that the Germans were already producing their own nuclear weapon. Nevertheless, Germany had seen a stagnation in scientific innovation in the years before and during the war. It would later be realized that although the Germans were trying to develop a nuclear weapon, they were not able to produce a bomb in the 1940s. One of the reasons for the lack of German scientific innovation was, of course, the Germans' enormous migration intelligence to Britain and neighboring countries as they fled the Nazi regime. In fact, the Fuchs family was not unique in their haste to flee their homeland. Many of the world's leading scientists - who had undoubtedly defined Germany's leadership in science in previous decades - had also fled their homeland in exile of Nazism (Williams 20).
While working with the pipe alloys, Fuchs' status as a security risk - more as a German than as a communist at the time - was waved because of his talents in analysis and research (Goodman 125). His most important tasks in the project included the theory of gas diffusion and the mathematics behind hydrodynamics. Ultimately, it was Fuchs' "phenomenal memory", his abilities as an "ideal analyst" and the ways he could "understand and remember in detail the most complex and difficult documents in his field" that made him a key member of the pipe alloys, and which influenced the future decision to hire him to work on the Manhattan Project in Los Alamos, in the laboratory that produced Little Boy and Fat Man, which were detonated on August 6 and 9, 1945 at Hiroshima and Nagasaki, respectively. (Williams 39).
For a general idea of World War II and their impact, see:https://www.yoair.com/blog/a-brief-history-and-timeline-of-the-world-wars/
Fuchs, however, had different motives. His communist loyalty encouraged him to contact the Soviet Embassy in London, at the suggestion of Jürgen Kuczynski (1904-97), because he wanted to share the information with them about the "Anglo-American" project he had started working on (Williams 60). In 1942, Sonia, Kaczynski's sister, who was heavily involved in this spy game, became Fuchs' contact person in Britain; officially made him a Soviet spy. Nevertheless, in many ways Fuchs legitimately believed that he was contributing to the greater good by sharing the secrets of the Tube Alloy project with the Soviets. He was motivated by the desire to see Nazism fail and the Axis powers defeated (Williams 15). Therefore, his goal of trumping the Nazi regime was very similar to that of the broader Allied initiates, but he is separated from the common goal because he refused to respect the constructions of political secrecy that prevented the transfer of information between Anglo-American efforts and Soviet efforts. , even though they had become allies in June 1941. Consequently, Fuchs continued to work as a Soviet spy when he was transferred to Los Alamos to work on the Manhattan Project, where he corresponded with Harry Gold (1910-72). However, according to Robert Chadwell Williams in Klaus Fuchs, Atom Spy (1987), although Fuchs made significant contributions to the creation of the bomb itself, there is little evidence that the specific information he provided to the Soviets was more than they already knew (7).

Consequently, in late January 1950, Fuchs dictated a lengthy statement acknowledging his work on behalf of the Soviet Union and then imprisoning him for nine years (Goodman 132). discoveries, but his aforementioned contributions to both the Tube Alloy Project and the Manhattan Project are marked by his attention to detail and appropriate analytical skills, which ultimately made him a valuable resource for both projects. Most significantly, however, his commitment to the projects represents his willingness to see the Nazi regime fail (Williams 20). More than anything else, Fuchs' motives for communicating with the Soviets were not done in vain, but rather in anticipation that the broader Allied efforts to defeat the Axis powers could be aided by the support of a Soviet nuclear weapon.

While many popular articles have appeared since the revelation of Fuchs' espionage crimes, such as Traitor Klaus Fuchs:He gave Stalin the A-bomb by Alan Moorhead, in an attempt to rogue Fuchs ', other accounts of Fuchs' motives suggest that he actually acted in pursuit of peace, as suggested by Robert Chadwell Williams ' Klaus Fuchs' Atom Spy (1987). In it, Williams does not refer to Fuchs as a criminal, but rather outlines the ways Fuchs had been influenced by his upbringing in Germany so that "fascism was the real enemy, and communism a moral parable of hope in a time of anxiety" (21). words, Fuchs' can be seen as a traitor, physicist or socialist, but the central point is that he was eventually a member and supporter of the Allied Front against the Axis powers, which had concluded that their only safe defense was nuclear weapons.
In any case, work on the Tube Alloy project continued, and the extent of the international allied efforts to trump Nazism reached a climactic point when the French confirmed the effectiveness of heavy water as a moderator in the nuclear process. By 1939, the Collège de France had been researching the "self-sustaining chain reaction that could be used as a source of industrial power". In other words, they had studied which moderators could be used to ensure that a nuclear reaction would emit the desired amounts of energy, by avoiding the absorption of said energy by the neutron particles during the process - which was the original problem observed with U 235 isotope (Clark 68). It was thus deduced that heavy water would be necessary for any kind of further nuclear development. The problem, however, was that only "one in every 5,000 hydrogen atoms consisted of the heavier isotope," which was necessary to produce the heavy water as a moderator, "had to be obtained in large quantities" (Clark 68).
For information on the fears associated with Nazism, please read the following article on the situation of Jews in Europe and the lack of many high-ranking officials to atone for their actions.
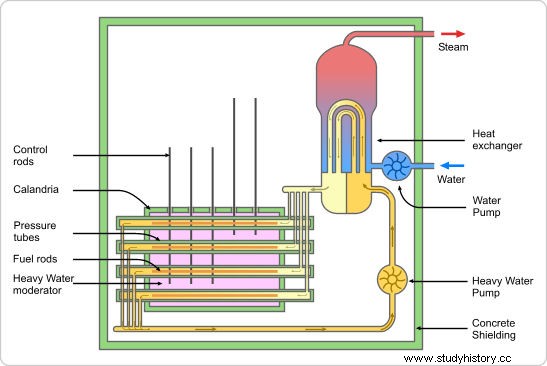
As such, a laboratory that produced heavy water, Norsk Hydro Company in Norway, had previously distributed small amounts of the resource throughout Europe (Clark 68). However, it was decided that the French lieutenant Jacques Allier (1940-1900) in 79 would secure a negotiation to transfer the Norwegian heavy water reserve to France. Within a few days of Allier's arrival in Oslo, he entered into an agreement to have a total of "one hundred and eighty-five kilos" of heavy water transferred to France (Clark 70). It is worth noting that the French before this initiative had not been interested in the special Allied efforts to defeat the Axis Powers using a nuclear weapon, as they had largely stuck to their individual research. As knowledge of the necessity of heavy water began to emerge in Britain and the United States, the need to secure an even broader political effort against Germany intensified. When France was officially occupied by the Axis forces in May 1940, it was decided by the summer that "the French would be incorporated into the British effort" to make a bomb (Clark 103).

Consequently, the Tube Alloy project would suppress the dense spectrum of French research, which would then be further summarized in the US initiative to develop the infamous Manhattan project. A completely different topic in itself, the Manhattan project represents the culmination of many years of research on the development of a nuclear weapon and is important for two main reasons. First, the British had been in constant communication with the Americans in an attempt to expand research on nuclear power, an initiative of President Franklin. Roosevelt D. officially approved in 1940 (one year before the inauguration of the pipe alloys), after being convinced of the seriousness of the situation in Europe in a letter written by Albert Einstein (1879-1955) and Leo Szilard (1898-1964), two leaders researchers at the time. Second, after the Japanese attack on Pearl Harbor on December 7, 1941, the Americans abandoned their previously neutral position and declared war on the Axis powers (Williams 16-7). Therefore, although a major motive for the Americans to join the Allied Atomic Initiative was because they knew they would not be able to challenge the Germans alone, they also joined because it was a broader, international necessity to defeat the Axis and restore world peace (Map 17).

That said, from the discovery of nuclear fission based on the first findings of Ernest Rutherford, the development of the Allied-based British Tube Alloy project that would eventually switch to the Manhattan project, and the usurping of French research and heavy water reserves by the British, it is clear that the originally scientifically motivated research in nuclear studies quickly became an international, allied effort to create a weapon of war that would secure the defeat of Germany and the Axis powers. In fact, as Cynthia C. Kelly demonstrates in The Birth of the Atomic Bomb in the words of its creators. Eyewitnesses and Historians "We believe that these considerations make use of atomic bombs for an early, unannounced attack on Japan that is not advisable. If the United States were to be the first to release this new means to. arbitrary destruction of humanity, she would sacrifice public support worldwide, accelerate the arms race and disrupt the possibility of reaching an international agreement on the future of control of such weapons ”(288).

Here it can be observed that even in the last stages of the development of the bomb, the scientists were not comfortable with the power of the weapon they had made. Yet the reason why, like Fuchs throughout his espionage plan, they remained loyal to their politically oriented research methods was because there was both a strong international push for Allied peace, and an understanding of how far the threat from Germany and the Axis powers extended. beyond the curiosity of scientific discovery. As such, the aforementioned factors define only a few ways in which the Allies joined forces - as initiated by a British operation - to form an international front against the Axis forces using an indisputably demonstrated nuclear weapon. Furthermore, the infamous example of the role Klaus Fuchs played in the development of the bomb, both politically and scientifically, shows that there was certainly a political motivation for introducing a memorandum on the use of nuclear energy on an international scale. The ultimate goal was therefore to manipulate science to secure political benefits that would re-establish and maintain peace on a global scale - perhaps something like the warning of a forerunner of the UN, which was founded in October 1945.

Japan was hard hit during World War II and the atrocities of nuclear power. For more information on Japanese culture, see:https://www.yoair.com/blog/anthropology-and-cultural-significance-of-japanese-art-history/
Works Cited
A Nuclear Reactor - Passing My Exams:Simple Exam Revision Notes for GSCE Physics , http://www.passmyexams.co.uk/GCSE/physics/nuclear-reactor.html.
Badash, Lawrence. Rutherford and Boltwood:Letters on Radioactivity . Yale UP, 1969.
Clark, Ronald W. The Birth of the Bomb:The Untold Story of Britain's Part in the Weapon that Changed the World . Foreword by Sir George Thomson, Charles Birchall and Sons, 1961.
Crew, David F. Hitler and the Nazis:A History in Documents . Oxford UP, 2006.
"Gaseous diffusion." United States Nuclear Regulatory Commission - Protecting People and the Environment , https://www.nrc.gov/reading-rm/basic-ref/glossary/gaseous-diffusion.html.
Goodman, Michael S. "Who is trying to keep it a secret and for whom and why:MI5-FBI relations and the Klaus Fuchs case." Journal of Cold War Studies , vol. 7, No. 2005, pp. 124-146. Academic Search Complete , http://dc153.dawsoncollege.qc.ca:2053/eds/pdfviewer/pdfviewer?vid=8&sid=7507252e-baeb-4fab-bdfc-b798f98ca3d9%40pdc-v-sessmgr04. Opened October 8, 2019.
Kelly, Cynthia C. The Manhattan Project:The Birth of the Atomic Bomb in the Words of Its Creators, Eyewitnesses, and Historians . Introduction by Richard Rhodes, Black Dog and Leventhal, 2007.
In short, Michael. The Columbia Guide to Hiroshima and the Bomb . Columbia UP, 2007.
Kortokikh, Alexander. 'Hydrodynamics and heat transfer in nuclear reactor. " Lecture 1:Nuclear Reactors , Department of Nuclear and Thermal Facilities.
Williams, Robert Chadwell. Klaus Fuchs, Atom Spy . Harvard UP, 1987.
Moorhead, Alan. "Traitor Klaus Fuchs:He gave Stalin the a-bomb." Saturday Evening Post , 224, No. 50, 1952, pp. 139-74. Masterfile Premier, http://dc153.dawsoncollege.qc.ca:2053/eds/pdfviewer/pdfviewer?vid=10&sid=7507252e-baeb-4fab-bdfc-b798f98ca3d9%40pdc-v-sessmgr04. Accessed October 8, 2019.
"Rutherford's Gold Foil Experiment." Khan Academy , Khan Academy, https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/history-of-atomic-structure/v/rutherfords-gold-foil-experiment.
Notes and Definitions
one. Alpha particles form the core of Helium. The atom is positively charged because the nucleus contains two positive charges and two neutral charges. Due to its nature, Rutherford knew that positively charged alpha particles moving near electrons would be slightly deflected because protons and electrons deflect each other (Khan Academy).
b. The screen that Rutherford used was one of zinc sulfide (Khan Academy).
c. Michael Kort describes the neutron as a "subatomic particle without charge and therefore in theory capable of penetrating a nucleus" in the Columbia Guide to Hiroshima and the Bomb (1998) (15).
d. Isotopes are alternative forms of a single element, whose properties change due to a change in the number of neutrons. To be mentioned later in the newspaper, U 235 has a shorter half-life, compared to U 238 , which means that it decays at a much faster rate, thus producing larger amounts of nuclear power (crew)
e. The heavy isotype of hydrogen.
[6] This would be a central point of contention in Fuchs' trial, as Michael S. Goodman describes in Who's Trying to Keep It a Secret for Who and Why:MI5 FBI Relations and the Klaus Fuchs Case , as a case that "raised many questions about MI5, in particular about the agency's control procedures", and where "such fears may have been reinforced by reports that the" socialist "Labor MP John Strachey was responsible for the British investigation into the security aspects of Fuchs cases ”(143).
Without a statement from the forces that wanted to ally with the Soviet Union the same year that Fuchs began working on the Tube Alloy project, and shortly before Fuchs himself became one of the most notorious Soviet spies in history, it was the Soviets who built a legitimate bomb.
g. Refers to the process of diffusion of uranium isotopes. This theory was crucial to understanding how U 235 , as an isotropic element, has a faster diffusion rate because it is a lighter element (Gaseous diffusion).
h. Studies the motion and forces of U 235 compared to U 238 .
I. Leader of the KDP (German Communist Party) and Soviet spy, although Fuchs was not aware of the latter.
j. Mainly through his research in Implosion Dynamics (Williams 68).
k. To ensure that U 235 fission chain reaction was not hindered by the absorption of neutron particles, a moderator was required. Heavy water has been shown to be an effective moderator for this purpose (A Nuclear Reactor).
l. Said Discovery would lead to the transformation of U 235 into Pu 239 (Plutonium) in the evolution of Little Boy and Fat Man (Map 17).
m. It is noteworthy that at this time the French had patented many of their discoveries, which constituted an obstacle to British efforts to use 'French discoveries', although various solutions were eventually devised (Clark 103).
Related:
Verdens dødeligste kriger:En beretning om historiske og moderne konflikter
